|
This section contains 87 words (approx. 1 page at 300 words per page) |
A measure of the acidity or alkalinity of a solution based on its hydrogen ion (H+) concentration. The pH of a solution is the negative logarithm (base 10) of its H+ concentration. Since the scale is logarithmic, there is a tenfold difference in hydrogen ion concentration for each pH unit. The pH scale ranges from 0 to 14 with 7 indicating neutrality ((H+)= (OH-)). Values above 7 indicate progressively greater alkalinity, while values below 7 indicate progressively increasing acidity.
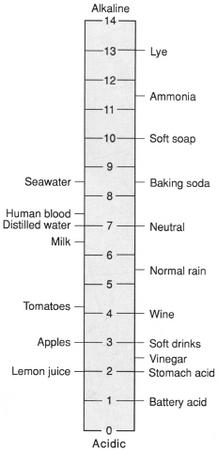 pH scale. (McGraw-Hill Inc. Reproduced by permission.)
pH scale. (McGraw-Hill Inc. Reproduced by permission.)
See Also
|
This section contains 87 words (approx. 1 page at 300 words per page) |


