|
This section contains 1,846 words (approx. 7 pages at 300 words per page) |

|
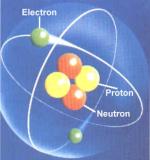
An electron is one of the three subatomic particles that make up an atom. The other two subatomic particles are protons and neutrons, which are found in the nucleus (center) of the atom. Electrons are found in the area outside the nucleus of an atom. They have a negative electrical charge (as opposed to protons, which are positively charged, and neutrons, which have no charge) and are quite small. An electron is only about 1/2,000 the size of a proton or a neutron. Protons and neutrons have masses of approximately 1 atomic mass unit (amu) each, whereas electrons only have a mass of .0006 amu (9.11 x 10- 28 g). The atomic number of an element is equal to the number of protons in one atom of the element. In a neutral atom, the number of electrons is equal to the number of protons, therefore, the atomic number also indicates the number of...
|
This section contains 1,846 words (approx. 7 pages at 300 words per page) |

|