|
This section contains 565 words (approx. 2 pages at 300 words per page) |

|
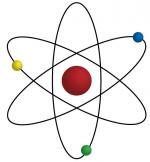
Atoms are the smallest particles of matter that have distinct physical and chemical properties. Each different type of atom makes up an element which is characterized by an atomic weight and an atomic symbol. Since the atomic theory was first proposed in the early nineteenth century, scientists have discovered a number of subatomic particles.
The development of the atomic theory traces its history to early human civilizations. To these people, change was a concept to ponder. Ancient Greek philosophers tried to explain the causes of changes in their environment, typically, chemical changes. This led them to propose a variety to ideas about the nature of matter. By 400 B.C., it was believed that all matter was made up of four elements including earth, fire, air and water. At around this time, Democritus proposed the idea of matter being made up of small indivisible particles. He called these particles...
|
This section contains 565 words (approx. 2 pages at 300 words per page) |

|